ABSTRACT
With the purpose to clarify the mechanism of the influence of external factors on the compatibility of polymers in a solvent, to study the effect of temperature,
concentration of polymer on the phase separation in two-phase systems dextran-PEG-water and dextran-PVP-water and PEG-C4O6H4Na2.
The thermodynamic parameters of the interaction between the components of the systems and the difference of these parameters (Δχ) was calculated and Δχ effect the
temperature dependence, dependence from concentration of additions was investigated. With increasing temperature and the concentration of polymer Δχ effect decreases,
simultaneously improving compatibility of polymers in aqueous solution.
Keywords: incompatibility of polymers, polymer-water two-phase system, phase separation.
PACS: 61.20.N,66.20.+d,82.60.Lf,61.25.Hq.
DOI:-
Received: 22.12.2022
AUTHORS & AFFILIATIONS
Baku State University, 23, Z. Khalilov st., Baku, AZ 1148, Azerbaijan
E-mail: Xaverhasanova2019@rambler.ru
Graphics and Images
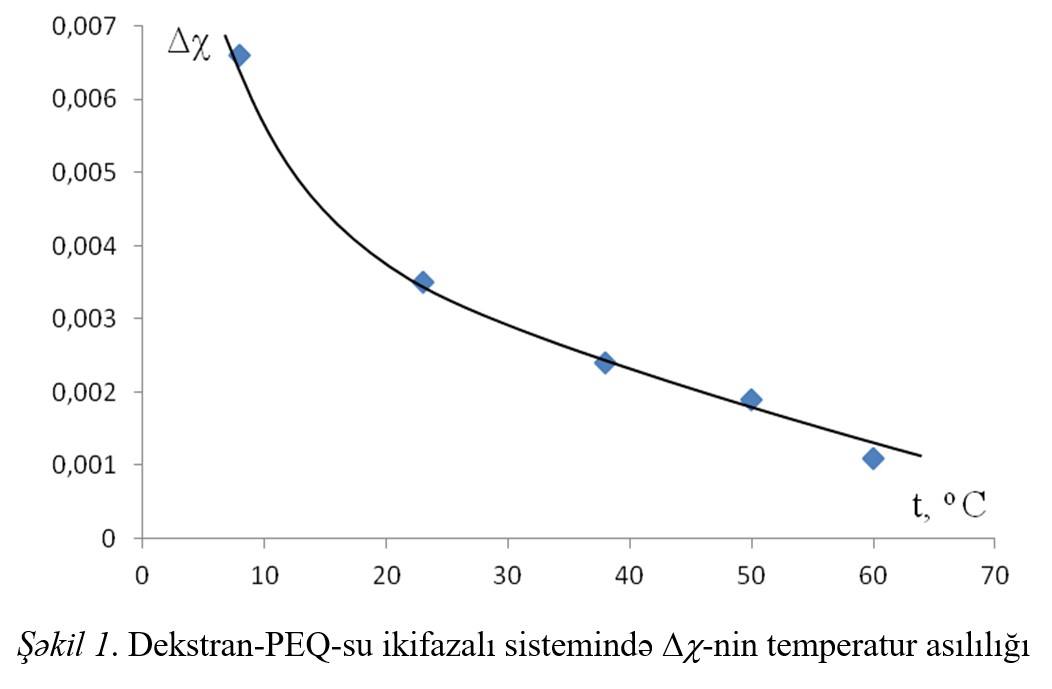
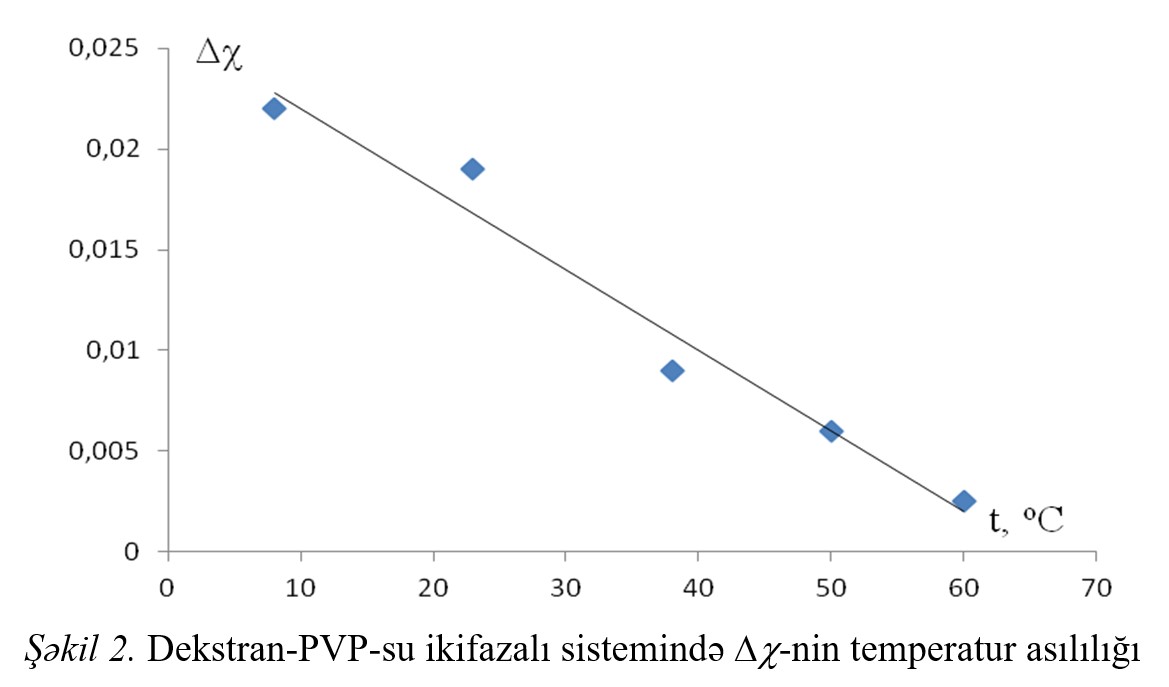
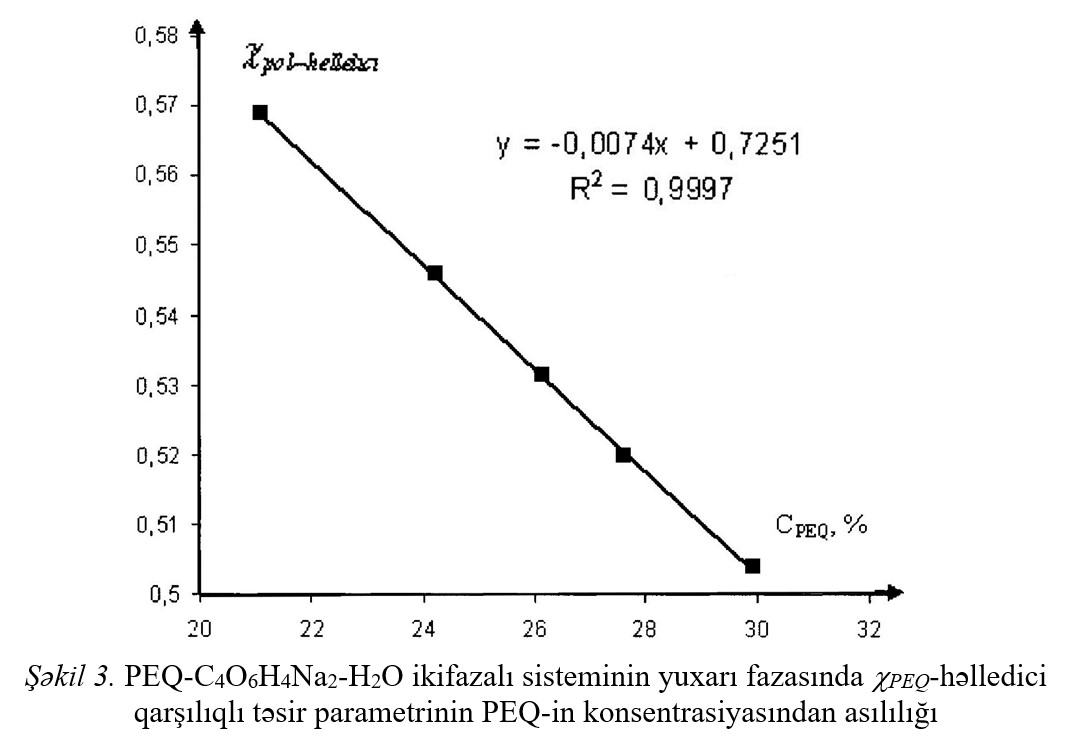
Fig.1 Fig.2 Fig.3
|
REFERENCIES
[1] В.Н. Кулезнев, Л.С. Крохина, Ю.Г. Оганесов, А.М. Злауск. Коллоидный журнал, 1971, т. 33, №1, с.98-105.
[2] Э.А. Масимов, Т.О. Багиров, Х.Т. Гасанова. Журнал изв. вузов «Химия и химическая технология», 2008, том 51, вып.2, с.123-126.
[3] Э.А. Масимов, Т.О. Багиров, У.Р. Саламова. Салех Азерпур. Вестник Бакинского Университета, серия физико-математических наук, 1998, № 2, с. 82-85.
[4] А.Е. Нестеров, Ю.С. Липатов. Термодинамика растворов и смесей полимеров. Киев, Наукова Думка, 1984, 300 с.
[5] Полимерные смеси, под редакции Д. Пола и С. Ньютен. М.: Мир, 1981, т.1,549 с.
[6] Л.З. Роговина, Г.Л. Слонимский. Успехи химии, 1977, т. 46, № 10, с.1871-1899.
[7] P.A. Albertsson, Partition of cell particles and macromolecules, 3rd Ed., Willey Press: New York, 1986, 396 p. v.2, № 1, p.90-100.
[8] A. Dobry, Boyer, F. Kawencki. Phase separation in polymer solution. Polymer Sci. 1947.
[9] P.J. Flory. Prediction of thermodynamic properties of polymer solutions. J. of Chemical Physics, 1941, v.9, p. 660-667.
[10] R. Scott. Prediction of thermodynamic properties of polymer solutions. J. of Chemical Physics, 1949, v.17, p.279-285.
[11] Ch. Hugelin, A. Dondos. Macromol. Chem., 1989, v.126, p.206-216.
[12] M.L. Huggins. Prediction of thermodynamic properties of polymer solutions. J. of Physical Chemistry, 1942, v.46, p.151.
[13] Minh le Van, T. Ohtsuka, H. Soyama, T. Nase. Polymer J., 1982, v.14, № 7, p.575-578.
[14] A. Robara, B. Patterson./ Macromolecules, 1977, v.10, № 5, p.1021-1033.
[15] G. Vandana, N. Sunil and C. Subhash. J. Polymer, 2002, v.43, № 11, p. 3387-3390.
[16] L. Zeman, B. Patterson. Macromolecules, 1972, v.3, № 4, p.513-516.
[17] B.Y. Zaslavsky. Aqueous Two-Phase Partitioning: Physical Chemistry and Bioanalytical Applications, Marcel Dekker, New York, 1994, 212 p.
[18] E.Ə. Məsimov., T.O. Bağırov, X.T. Həsənova. PEQ-qeyri-üzvi elektrolit sistemlərinin hal diaqramlarının termodinamik analizi. Bakı Universitetinin xəbərləri, 2004, № 3, səh. 97-102
[19] A.Ə. Həsənov, X.T. Həsənova, S.R. Bağırova. Influense of some salts on binodaline of biphasic systems formed by noninoic polymers AJP Fizika 2022 vol.XXVIII № 1, sec.En. 3-7.
|
