ABSTRACT
A naturally occurring biopolymer known as silk fibroin (SF) has several distinctive qualities that make it an ideal vehicle for the delivery of drugs and a variety of
therapeutic agents. It has been demonstrated that drugs and biomolecules can be successfully delivered via SF matrices. In this study homogenous SF nanoparticles (SFNP)
obtained with a size ranging between 171-207 nm. SFNPs were stabilized with GA (glutaraldehyde) which is known as a cross-linking agent was used to stabilize the SFNPs.
The stabilized SFNPs were consistent for 4 days. Circular dichroism (CD) and Fourier-transform infrared spectroscopy (FTIR) studies revealed random-coil to beta structure
transition with beta structure reaching up to 59 %. Observed birefringence of Congo red staining in a polarized microscope indicates beta amyloid formation. Thus, SFNPs
composed of amyloid nanoparticles.
Keywords: silk fibroin, silk nanoparticles, cross linker, amyloids.
PACS: 87.64.−t; 87.64.Cc
DOI:-
Received: 20.07.2023
AUTHORS & AFFILIATIONS
Institute of Biophysics, Ministry of Science and Education Republic of Azerbaijan Z. Khalilov, 117, Baku, AZ 1171
E-mail: mesimlia@mail.ru; basira.qasimli.usubali@bsu.edu.az
Graphics and Images
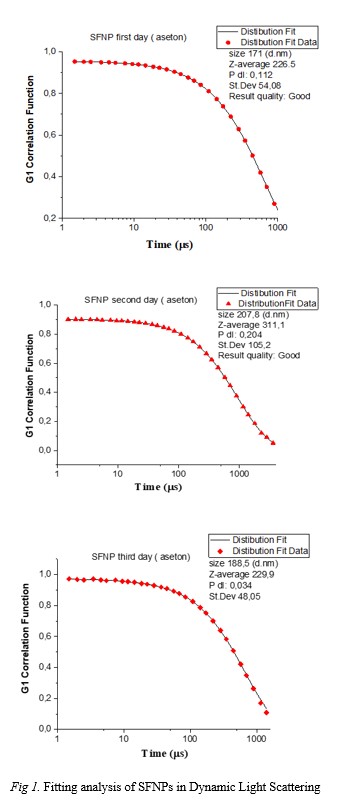
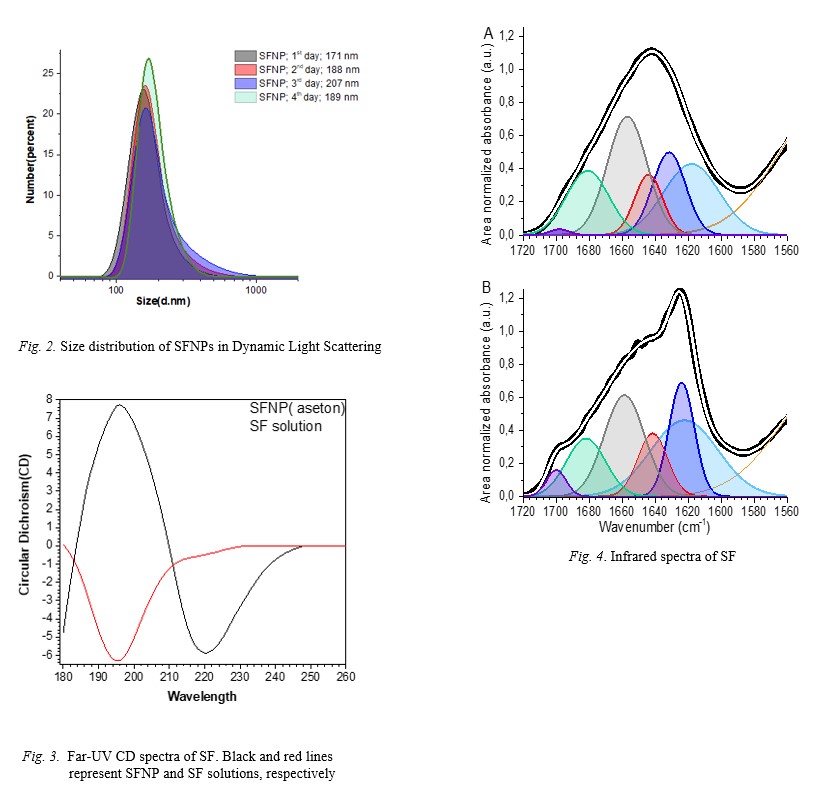
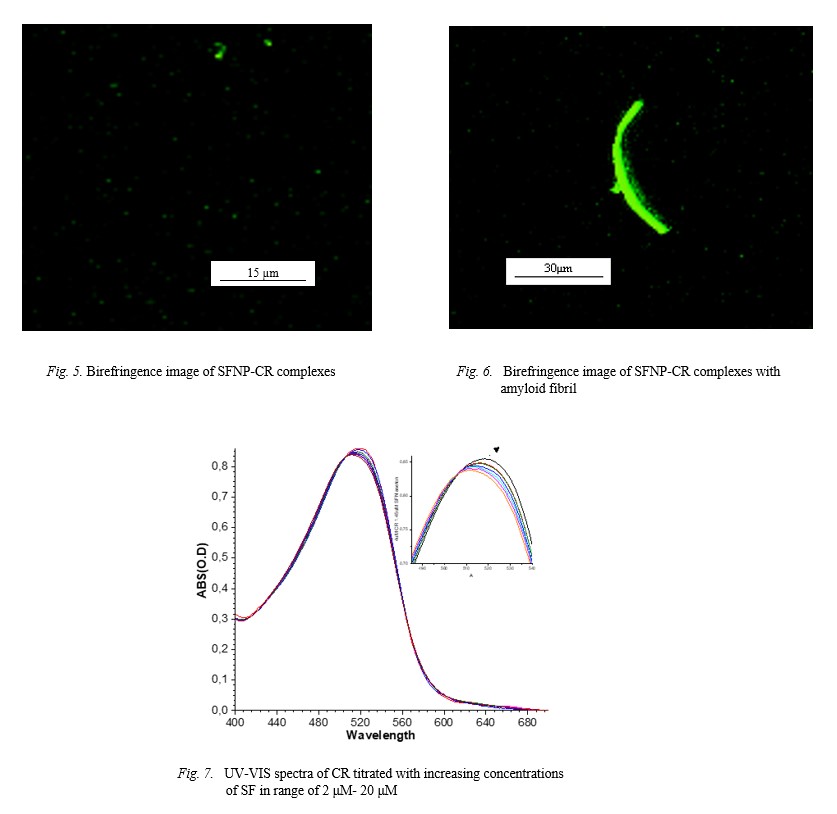
Fig.1 Fig.2 Fig.3
|
REFERENCIES
[1] B. Crivelli, S. Perteghella, E. Bari, M. Sorrenti, G. Tripodo, T. Chlapanidas, M. Torre. Silk nanoparticles: from inert supports to bioactive natural carriers for drug delivery. Soft matter, 2018, 14(4), 546–557.
[2] C.L. Craig. Evolution of arthropod silks, Annu Rev Entomol 42, 1997, 231-67.
[3] K. Yamaguchi, Y. Kikuchi, T. Takagi, A. Kikuchi, F. Oyama, K. Shimura, S. Mizuno. Primary structure of the silk fibroin light chain determined by cDNA sequencing and peptide analysis, J Mol Biol 210(1) (1989) 127-39.
[4] H. Okamoto, E. Ishikawa, Y. Suzuki. Structural analysis of sericin genes. Homologies with fibroin gene in the 5' flanking nucleotide sequences, J Biol Chem. 1982, 257(24), 15192-9.
[5] D.N. Rockwood, R.C. Preda, T. Yücel, X. Wang, M.L. Lovett, D.L.Kaplan. Materials fabrication from Bombyx mori silk fibroin. Nature protocols, 2011, 6(10), 1612–1631.
[6] F. Mottaghitalab, M. Farokhi, M.A. Shokrgozar, F. Atyabi, H. Hosseinkhani. Silk fibroin nanoparticle as a novel drug delivery system, Journal of Controlled Release, 2015, (Vol. 206).
[7] R. Mehravar, M. Jahanshahi, N. Saghatoleslami. Fabrication and evaluation of Human Serum Albumin (HSA) nanoparticles for drug delivery application. International Journal of Nanoscience, 2009, 8(3).
[8] W. Huang, S. Ling, C. Li, F.G. Omenetto, D.L. Kaplan. Silkworm silk-based materials and devices generated using bio-nanotechnology, Chem. Soc. Rev. 2018, 47, 6486–6504.
[9] L. Ragona, O. Gasymov, A.J. Guliyeva, R.B. Aslanov, S. Zanzoni, C. Botta, H. Molinari. Rhodamine binds to silk fibroin and inhibits its self-aggregation, Biochim. Biophys. Acta, Proteins Proteomics, 2018, 1866, 661–667.
[10] W.E. Klunk, R.F. Jacob, R.P. Mason. Quantifying amyloid beta-peptide (Abeta) aggregation using the Congo red-Abeta (CR-abeta) spectrophotometric assay. Anal Biochem. 1999, 266(1):66-76.
[11] O.I. Antimonova, N.A. Grudinina, V.V. Egorov, D.S. Polyakov, V.V. Iljin, M.M. Shavlovsky. Interaction of the dye Congo red with fibrils of lysozyme, beta2- microglobulin and transthyretin, Tsitologiia, 2016, 58 156–163.
[12] A.J. Howie, D.B. Brewer, D. Howell, A.P. Jones. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Laboratory investigation; a journal of technical methods and pathology, 2008, 88(3), 232–242.
[13] B. Ranjbar, P. Gill. Circular Dichroism Techniques: Biomolecular and Nanostructural Analyses- A Review. Chemical Biology & Drug Design, 2009, 74: 101-120.
[14] E.I. Yakupova, L.G. Bobyleva, I.M. Vikhlyantsev, A.G. Bobylev. Congo Red and amyloids: history and relationship, Biosci Rep. 2019, Jan 15;39(1)
|
