ABSTRACT
The influence of a number of alkaline salts (NaCl, KCl, CaCl2 and KBr) on the gelation processes in aqueous agarose solution was studied by spectrophotometric
method. The results of the study show that different anions have different effects on the gel. Among the salts where the anion is stable, KCl has the least effect on the
gel formation and gel melting temperature, and among the salts where the cation remains stable, KBr has the least effect. The detected patterns are related to the influence
of these salts on the structures of water, the medium where the gel is formed. The influence of inorganic salts on the structure of water is explained by the ability of
ions to polarize water, which depends on the size, charge and charges surface density of the ion.
Keywords: agarose, alkaline salts, arrival temperature, melting temperature, hysteresis
PACS: 77.22.Ej, 64.75 Bc, 31.70. Dk, 61.70 Og
DOI:-
Received:
AUTHORS & AFFILIATIONS
Baku State University, 23, Z. Khalilov st., Baku, AZ 1148, Azerbaijan
E-mail: aynurasadova19@gmail.com
Graphics and Images
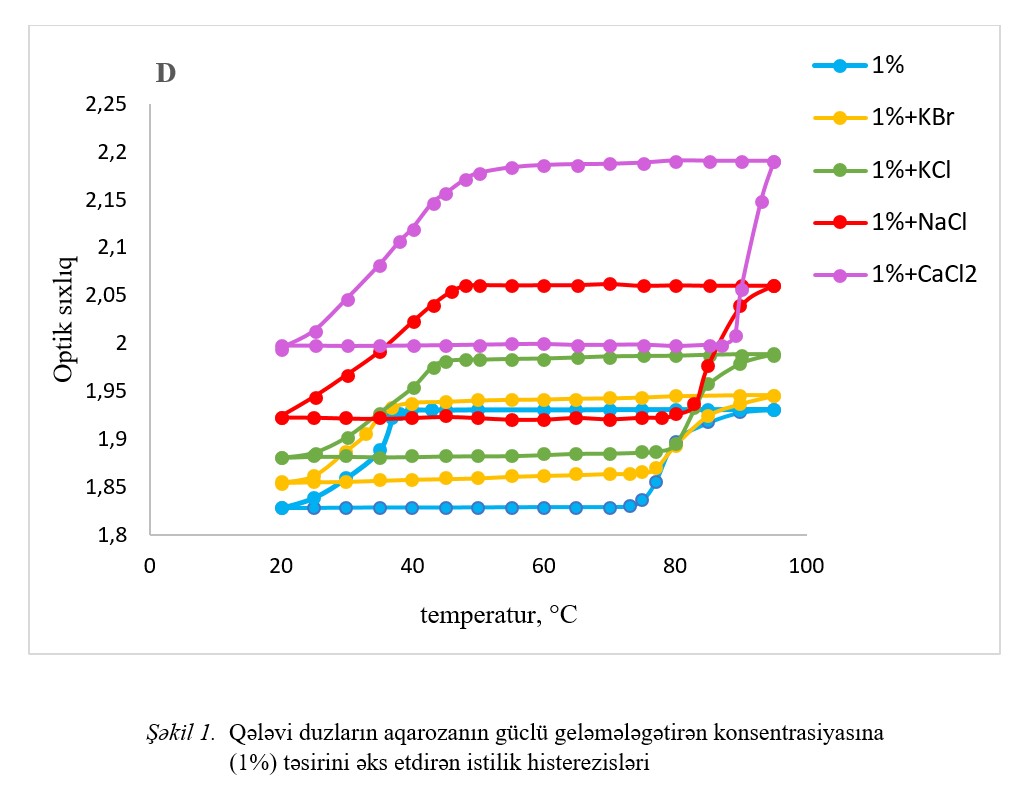
Fig.1
|
REFERENCIES
[1] Elena Varoni, Matilde Tschon, Barbara Palazzo, Paola Nitti, Lucia Martini, Lia Rimondini. Agarose Gel as Biomaterial or Scaffold for Implantation Surgery: Characterization, Histological and Histomorphometric Study on Soft. Connective Tissue Research, 2012; 53(6): 548–554
[2] M. Tako, S. Nakamura. Gelation mechanism of agarose, Carbohydate Research, 1988, 180 (2), 277-284.
[3] Arif Selcuk Ogrenci, Onder Pekcan, Selim Kara &Ayse Humeyra Bilge. Mathematical Characterization of Thermoreversible Phase Transitions of Agarose Gels. Journal of Macromolecular Science, Part B Physics 2018.
[4] Emiliano Fernandez, Daniıel Lopez, Carmen Mijangos, Miroslava Duskova-Smrckova, Michal Ilavsky, Karel Dusek. Rheological and Thermal Properties of Agarose Aqueous Solutions and Hydrogels. Journal of Polymer Science: Part B: Polymer Physics, 2008, vol. 46, 322–328.
[5] C. Viebke, L. Piculell, S. Nilssont. On the Mechanism of Gelation of Helix-Forming Biopolymers. Macromolecules 1994, 27, 4160–4166.
[6] M. Djabourov, J. Leblond, P. Papon. Gelation of acqueous gelatin solutions. II. Rheology of the sol–gel transition. J. Phys. Fr. 1988, 49, 333–343.
[7] E.Ə. Məsimov, A.R. İmaməliyev. Polimer gellərin fiziki xassələri. Bakı-2014.
[8] A.H. Asadova and E.A. Masimov. The solution-gel phase transition in aqueous solutions of agarose Modern Physics Letters B, vol. 35, No. 8, 2021, 2150147 (7 pages)
[9] P.L. Indovina, E. Tettamanti, M.S. Micciancio Giammarinaro and M.U. Palma. Thermal hysteresis and reversibility of gel–sol transition in agarose–water systems: The Journal of Chemical Physics 1979, 70, 2841.
[10] M. Watase, K. Nishinari. The Effect of Sodium Thiocyanate on Thermal and Rheological Properties of kappa-Carrageenan and Agarose Gels. Carbohydrate Polymers 11, 1989, 55-66, 269-284
[11] Struther Arnott, A. Fulmer, W.E. Scott. The Agarose Double Helix and Its Function in Agarose Gel Structure. J. Mol. Biol., 1974, 90.
[12] Lennart Picdell and Svante Nilsson. Anion-Specific Salt Effects in Aqueous Agarose Systems. 1. Effects on the Coil-Helix Transition and Gelation of Agarose. J. Phys. Chem. 1989, 93, 5596-5601.
|
