ABSTRACT
The glass transition and crystallization processes of Se95Te5 HG system were studied using a differential scanning calorimeter in non-isothermal mode
with a heating rate of 5,10,15,20 K/min. The glass transition, crystallization, and melting temperatures were determined and the reduced value of the glass transition
temperature (Trg), Hruby coefficient (Kgl), brittleness coefficient (Fi), and Avrami coefficients (n and m), as well as the activation energy of the glass transition and
crystallization processes (140.24 kС/mol, 95.11 kС/mol) was calculated. It has been shown that the composition of XSHY Se95Te5 has a high degree of
glass-forming properties. It was determined that the crystallization mechanism (n = 2.51 m = 1.9) corresponds to volumetric nucleation accompanied by two-dimensional growth.
Keywords: chalcogenide, glass transition temperature, crystallization temperature, brittleness factor.
DOI:10.70784/azip.2.2024107
Received: 19.01.2024
AUTHORS & AFFILIATIONS
Institute of Physics Ministry of Science and Education Republic of Azerbaijan, AZ 1143, Baku, Azerbaijan, 131, H.Javid ave.
E-mail: seva_atayeva@mail.ru
Graphics and Images
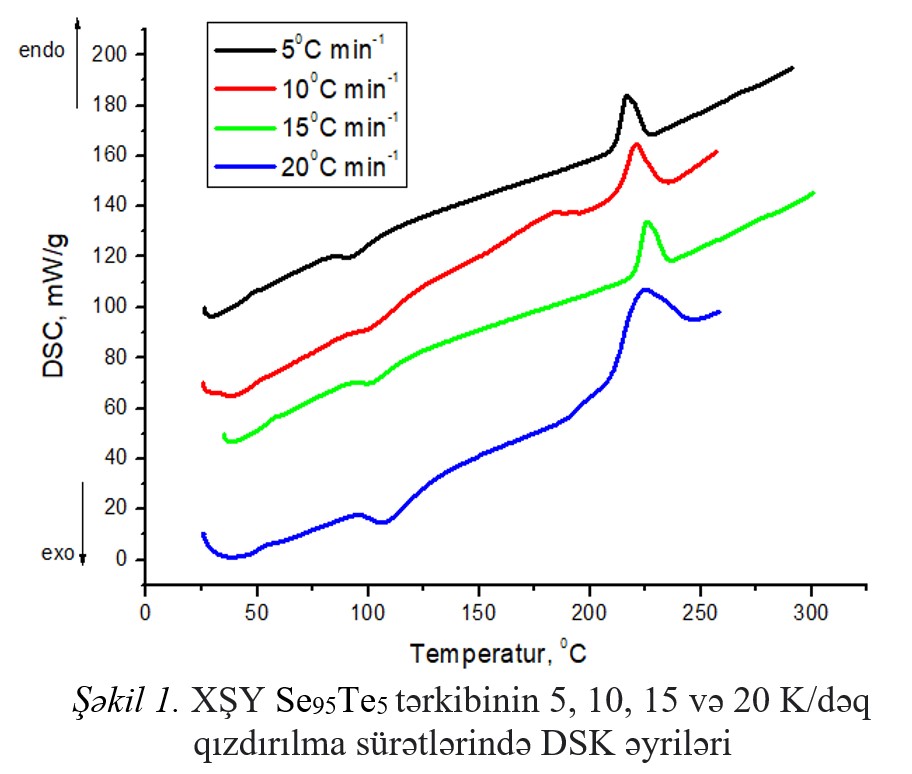
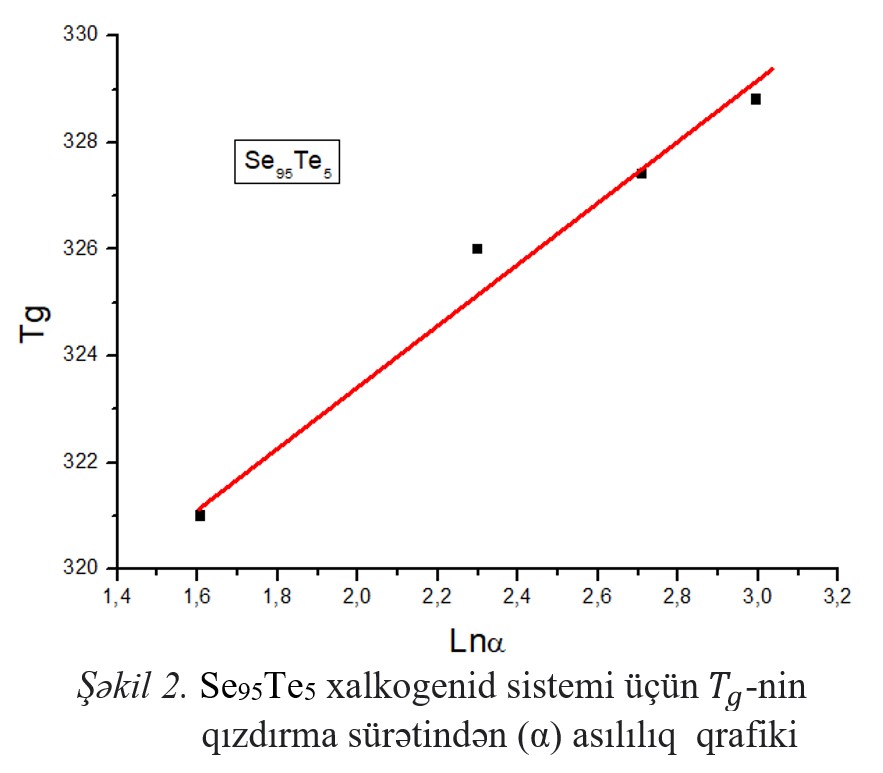
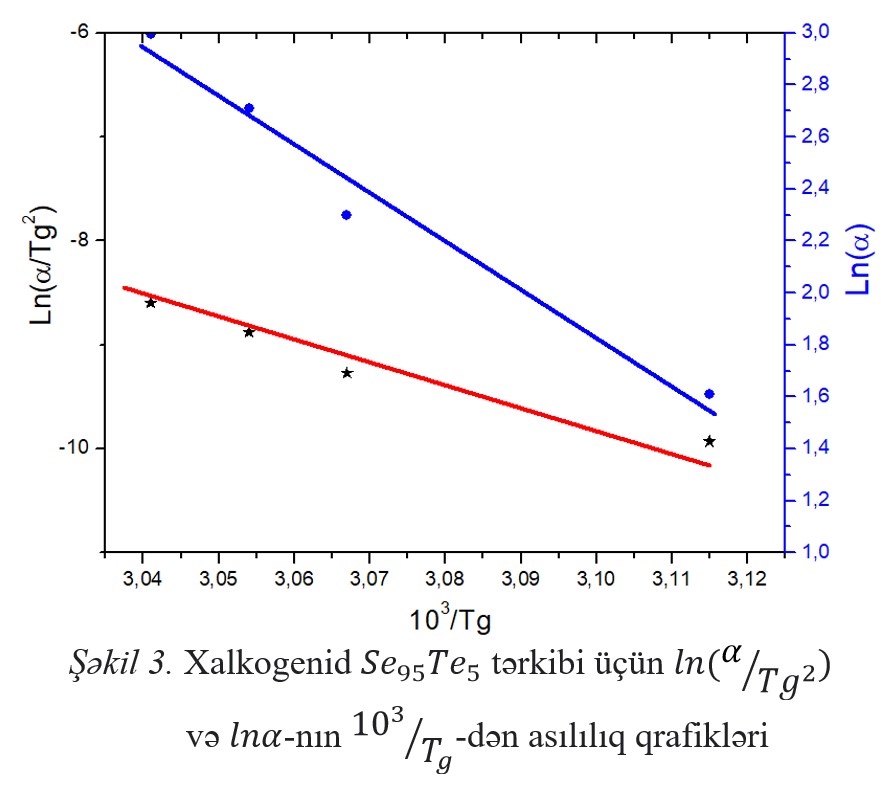
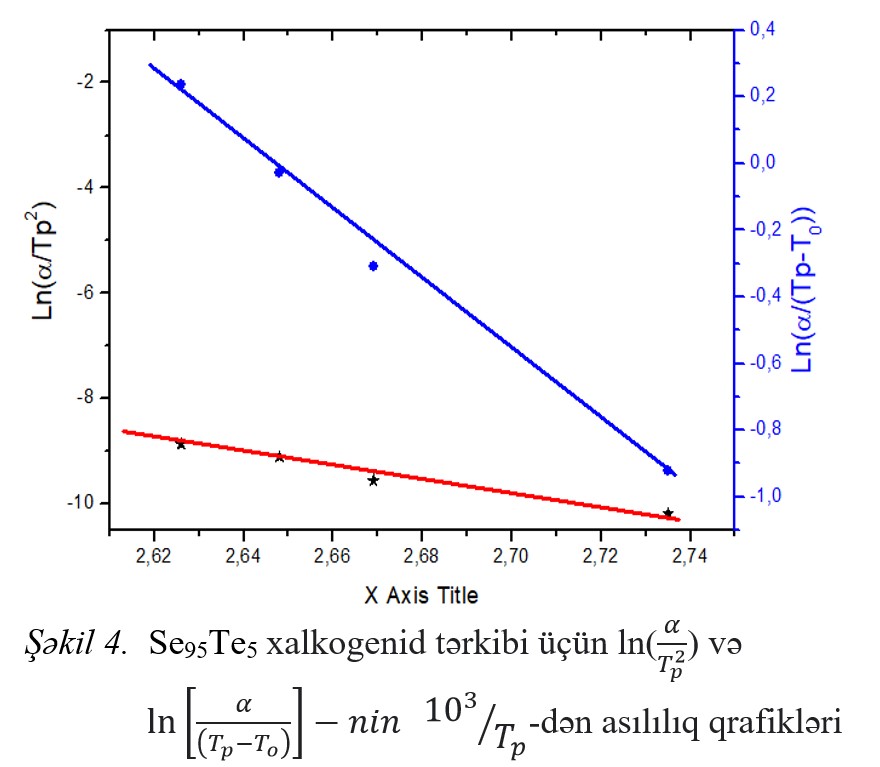
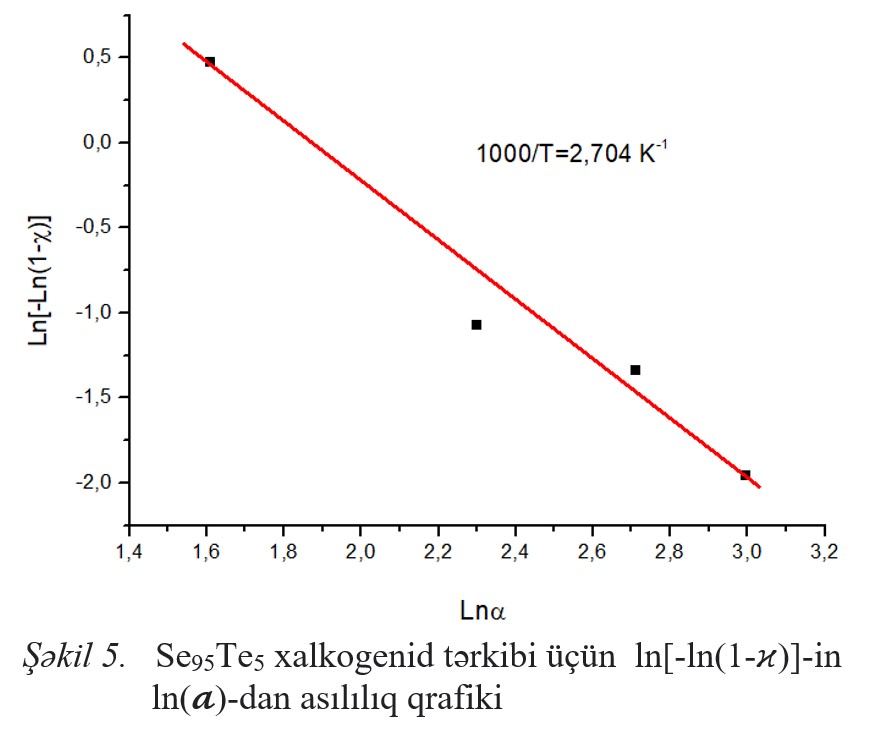
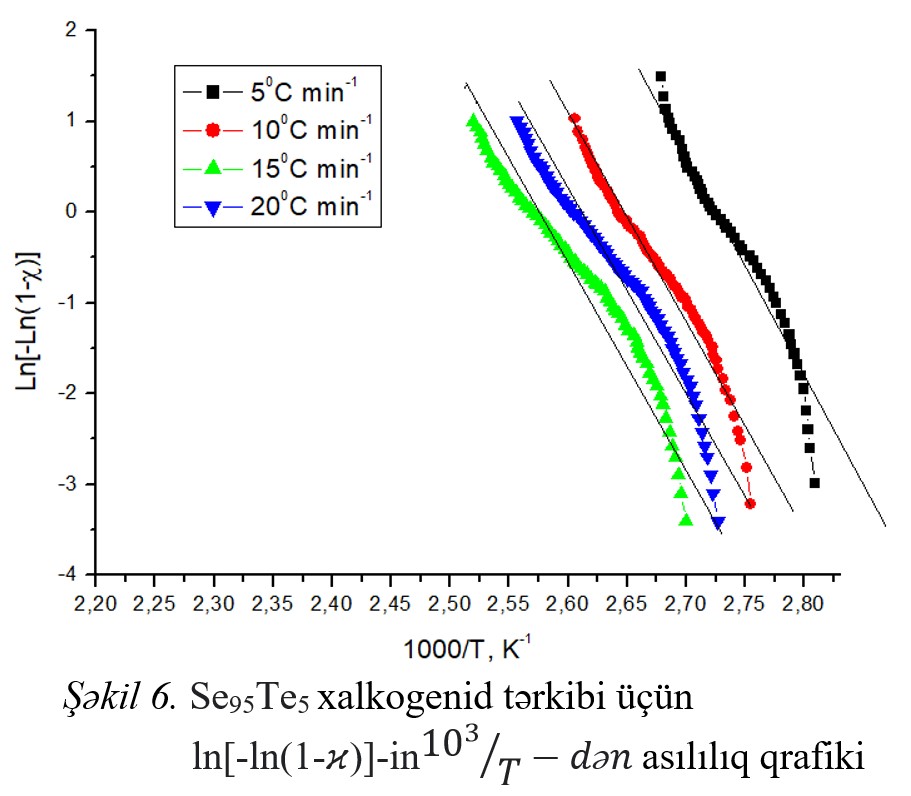
Fig.1 Fig.2 Fig.3 Fig.4 Fig.5 Fig.6
|
REFERENCIES
[1] B.J. Eggleton. Chalcogenide photonics: Fabrication, devices and applications. Introduction, Opt. Express 2010, vol. 18, № 25, p. 26632.
[2] G. Tao, H. Ebendorff-Heidepriem, A.M. Stolyarov, S. Danto, J.V. Badding, Y. Fink, J. Ballato, A.F. Aboyraddy. Infrared fibers, Adv.Opt. Photonics 7, 2015, 379 e 458.
[3] W.H. Kim,V.Q. Nquyen, L.B. Shaw, L.E. Busse, C. Florea, D.J. Gibson, R.R. Gattass, S.S. Bayya, F.H. Kung, G.D. Chin, R.E. Miklos, I.D. Aggarwal, J.S. Sanghera. Recent progress in chalcogenide fiber technology at NRL, J. Non-Cryst. Sol. 431, 2016, 8e15.
[4] N. Mehta Applications of chalcogenide glasses in electronics and optoelectronics: a review J. Sci. Ind. Res., 65 (10), 2006, pp. 777-786.
[5] B. Bureau, X.H. Zhang, F. Smektala, J.L. Adam, J. Troles, H.L. Ma, C. Boussard-Pledel, J. Lucas, P. Lucas, D. Le Coq, M.R. Riley, J.H. Simmons. Recent advances in chalcogenide glasses J. Non-Cryst. Solids, 345, 2004, pp. 276-283.
[6] X. Zhang, H. Ma, J. Lucas. Applications of chalcogenide glass bulks and fibres J. Optoelectron. Adv. Mater., 5 (5), 2003, pp. 1327-1333.
[7] S. Raoux, W. Welnic, D. Ielmini. Phase change materials and their application to nonvolatile memories Chem.Rev.,110 (1), 2010, p.240-267.
[8] A. Kumar, R. Misra, S.K. Tripathi. Effect of partial crystallization on the photoconductive behaviour of amorphous thin films of Se75Te25. Semicond Sci Technol. 1989;4:1151–5
[9] H.E. Kissinger. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956, 57, 217–21.
[10] H.E. Kissinger. Reaction kinetics in differential thermal analysis. Anal Chem. 1957, 29,1702–6.
[11] J.A. Augis, J.E. Bennett. Calculation of the Avrami parameters forheterogrneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978, 13, 283–92.
[12] K. Matusita, T. Komatsu, R. Yokota. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J Mater Sci. 1984, 19, 291–6.
[13] Y.Q. Gao, W. Wang. On the activation energy of crystallization in metallic glasses. J Non-Cryst Solids. 1986, 81, 129–34.
[14] C.T. Moynihan, A.J. Easteal, J. Wilder, J.Tucker. Dependence of the glass transition temperature on heating and cooling rate. The Journal of Physical Chemistry. 1974, 78, 2673-2677.
[15] J.P. Larmagnac, J. Grenet, P. Michon. Glass transition temperature dependence on heating rate and on ageing for amorphous selenium films. Journal of Non-Crystalline Solids. 1981, 45(2), 157-168.
[16] S.O. Kasap, C. Juhasz. Kinematical transformations in amorphous selenium alloys used in xerography. JournalofMaterialsScience. 1986, 21, 1329-1340.
[17] W. Kauzmann. The nature of the glassy state and the behavior of liquids at low temperatures. Chemical Reviews. 1948, 43, 219-256.
[18] A. Hrubý. Evaluation of glass-forming tendency by means of DTA. Czechoslovak Journal of Physics. 1972, 22, 1187-1193.
[19] M. Avrami. Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941, 9, 177–84.
[20] M. Lasocka.The effect of scanning rate on glass transition temperatureof splat-cooled Te85Ge15. Mater Sci Eng. 1976, 23, 173–7.
[21] Physical Review B. 1993, 47, 2882-2885.
[22] W.A. Johnson, R.F. Mehl. Reaction kinetics in processes ofnucleation and growth. Trans Am Inst Min Metall Eng. 1939, 135, 416–58.
[23] M. Avrami. Kinetics of phase change. I. General theory. J ChemPhys. 1939, 7, 1103–12.
[24] M. Avrami. Kinetics of phase change. II. Transformation-timerelations for random distribution of nuclei. J Chem Phys.1940, 8, 212–24.
[25] K. Matusita and S. Sakka. “Kinetic Study of the Crystallization of Glass by Differential Scanning Calorimetry, Phys. Chem. Glasses 20, 1979, Xl.
|
