ABSTRACT
In the article, the glass transition processes of the Sb80Se20 chalcogenide composition were studied by the differential scanning calorimetry (DSC)
method depending on the heating rate in the temperature range of t=20÷300°C. It was determined that as a result of the increase in the heating rate (q) (in the range
of q=2.5-17.5 °C/min), the "fragility" coefficient (m) undergoes a slight decrease, which leads to a partial increase of thermal stability (∆T) and a weak increase of
glass-forming ability [or Hruby number (Hr)], thermal stability parameters (Hʹ, S).
Keywords: chalcogen, glass, amorphous, fragility coefficient.
DOI:10.70784/azip.2.2024323
Received: 11.09.2024
Internet publishing: 18.09.2024
AUTHORS & AFFILIATIONS
Institute of Physics named after H.M.Abdullayev of the Ministry of Science and Education Republic of Azerbaijan, 131 H.Javid ave. Baku, AZ-1143, Azerbaijan
E-mail: Rahim-14@mail.ru
Graphics and Images
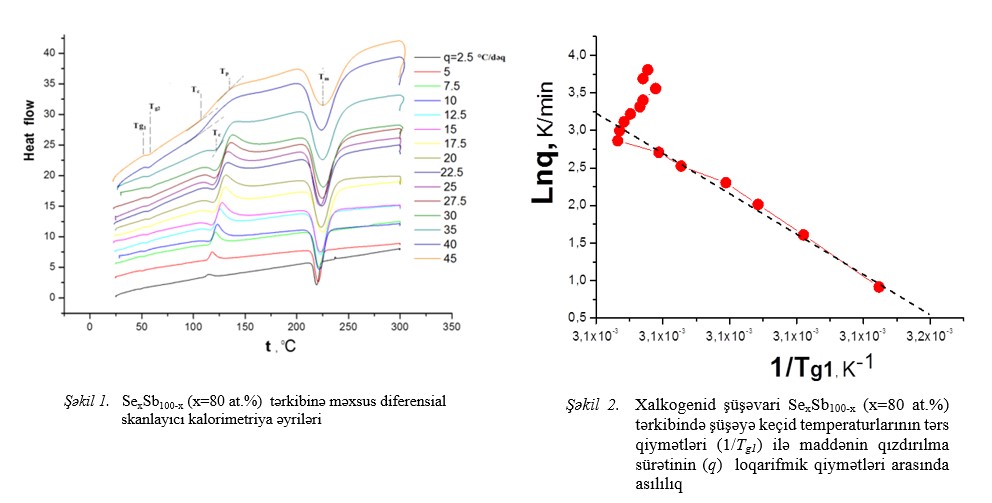
Fig.1-2
|
[1] P.G. Debenedetti, F.H. Stillinger. Nature 410, 2001, 259-267.
[2] C. A. Angell. "Formation of Glasses from Liquids and Biopolymers". Science. 1995, 267 (5206): 1924–1935.
[3] K. Tanaka. Fragility of 1D floppy materials. Phys. Stat. Sol. B 2000206, 2020.
[4] K. Tanaka. Amorphous Chalcogenide Semiconductors and Related Materials, K. Tanaka, K. Shimakawa, Springer Nature Switzerland AG, 2021, 310 p.
[5] Yoshifumi Sakaguchi and Kozaburo Tamura. Z. Phys. Chem. 235, 2021, 189–212.
[6] M. Wilson, P.S. Salmon. Network topology and the fragility of tetrahedral glass-forming liquids, Phys. Rev. Lett, 103, 2009, 157801.
[7] D.L. Sidebottom. Fifty years of fragility: A view from the cheap seats. J. Non-Cryst. Solids 524, 2019. 119641.
[8] N. Mehta, R.S. Tiwari, A. Kumar. Materials Research Bulletin 41, 2006, 1664–1672.
[9] D. Dimitrov, D. Tzocheva, D. Kovacheva. Thin Solid Films 323, 1998, 79–84.
[10] Roman Svoboda, Jan Prikryl, Alexander V. Kolobov, Milos Krbal. Ceramics International 48, 2022, 17065–17075.
[11] R. Böhmer, K.L. Ngai, C.A. Angell and D.J. Plazek. J. Chern. Phys. 99, 1993, 4201-4209.
[12] E. Donth. The Glass Transition Relaxation Dynamics in Liquids and Disordered Materials, Springer-Verlag Berlin Heidelberg, Germany, 2001, 411p
[13] N. Bulat Galimzyanov, Anatolii V. Mokshin. Journal of Non-Crystalline Solids 570 (2021) 121009
[14] C.T. Moynihan, A.J. Wilder, J. Tucker. J. Phys. Chem. 78, 1974, 2673-2677.
[15] J. Kieran. Crowley, George Zografi// Thermochimica Acta 380, 2001, 79-93.
[16] A. Hruby. “Evaluation of Glass-Forming Tendency by Means of DTA,” J. Physics B, vol. 22, 1972, p. 1187.
[17] M.M. Soraya, Fouad Abdel-Wahab, A.A. Elamin, E.R. Shaaban, N.N. Ali Karrar. Structural and thermal characteristics of Ge30−xSbxTe10Se60 (0≤x≤20) glasses for electronic devices, Journal of Thermal Analysis and Calorimetry 148, 2023, pp. 5927–5942.
[18] W. Kauzmann. The nature of the glassy state and the behavior of liquids at low temperatures. Chem. Rev. 43, 1948, pp. 219–256.
[19] L.-M. Wang, Z. Li, Z. Chen, Y. Zhao, R. Liu, Y. Tian. Glass transition in binary eutectic systems: Best glass-forming composition. J. Phys. Chem. B 114, 2010, pp. 12080–12084.
[20] E. Hempel, G. Hempel, A. Hensel, C. Schick, E. Donth. J. Phys. Chem. B 104, 2000, 2460-2466.
[21] S. Capaccioli, G. Ruocco, F. Zamponi. J. Phys. Chem. B 112, 2008, 10652-10658.
[22] R. Richert, C.A. Angell. J. Chem. Phys. 108, 1998, 9016-9026.
[23] C.A. Angell, B.E. Richards, V. Velikov: J. Phys. Condens. Matter 11, 1999, A75-A94.
[24] C.A. Angell. J. Non. Cryst. Solids 73, 1985, 1-17.
[25] J.J.M. Ramos, N.T. Correia, H.P. Diogo, C.Alvarez, T.A. Ezquerra, J. Non. Cryst. Solids 351, 2005, 3600-3606.
[26] Sabyasachi Sen, Jacob Lovi, J. Non. Cryst. Solids, vol.638, 2024, 123060.
|
