ABSTRACT
In this study, we aimed to investigate the temperature effects of a low-power laser on a tissue-like material, namely muscle phantom. To do this, we used thermistor
temperature sensors to measure the temperature of the material at various points while it was exposed to the laser. We found that the temperature of the material increased
with increasing duration of exposure to the laser, however, the maximum temperature increase was 1.7 degrees Celsius for 80 seconds, which is not harmful to tissues. Next,
we applied this temperature difference information to the heat transfer equation, a mathematical model that describes how heat is transferred from one body to another. By
using the measured temperature difference values and known variable such as the material's specific heat capacity, we were able to calculate the amount of energy per 1 gram
produced by laser light at different distances and durations. We found that the maximum energy produced was 8.4 J for 80 s or a fluence of 0.105 J/cm2 per second. This
information is important because it can help us understand how the laser's energy is absorbed by the tissue-like material and how it affects the material's properties and
structure. It can also provide insight into the potential therapeutic effects of low-level laser therapy, a non-invasive treatment that uses lasers to stimulate healing and
reduce pain and inflammation in the body, and for determining appropriate treatment doses for various diseases.
Keywords: Tissue-Like Material, Low Power Laser, Temperature Effects, NTC-Type Thermistor Temperature Sensor, Heat Transfer Equation, Specific Heat Capacity, Energy Calculation.
PACS:
DOI:-
Received: 29.03.2023
AUTHORS & AFFILIATIONS
1. Department of Physics, Gebze Technical University, 41400, Kocaeli, Turkey
2. Medical Metrology Laboratory, TUBITAK National Metrology Institute (TUBITAK UME), 41470, Kocaeli, Turkey
E-mail: hokandurmus@gtu.edu.tr, smirhasan@gtu.edu.tr
Graphics and Images

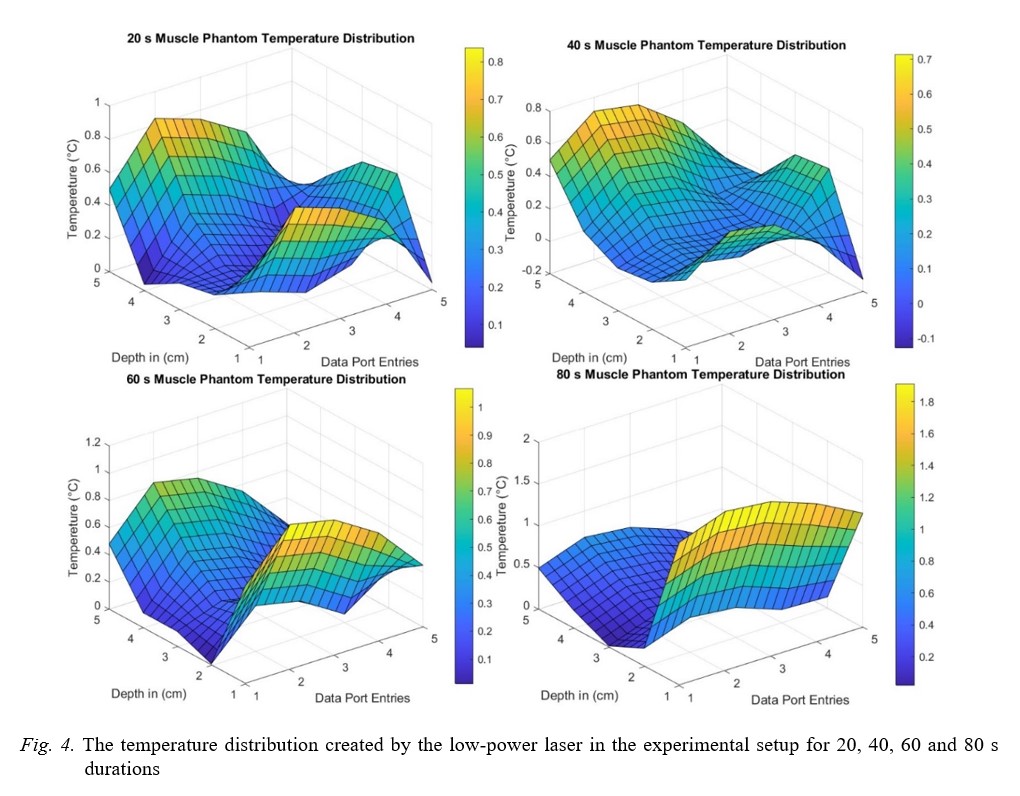
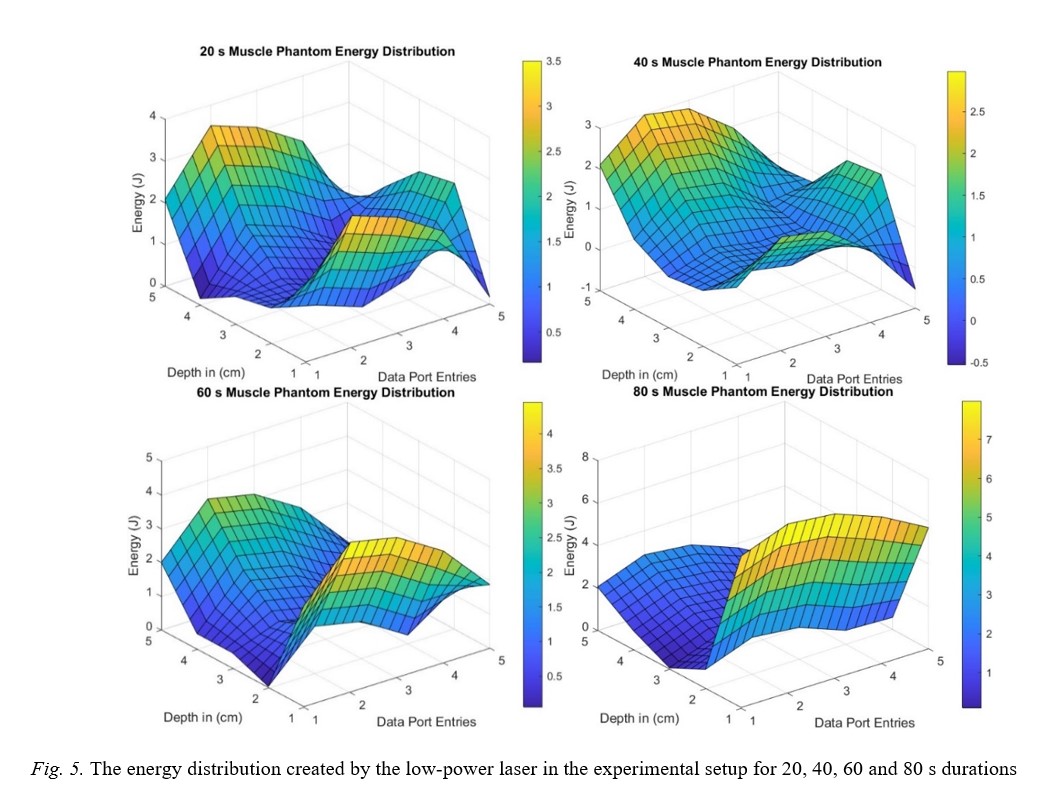
Fig.1 Fig.2 Fig.3 Fig.4 Fig.5
|
REFERENCIES
[1] C. K. McGarry, L.J. Grattan, , A. M. Ivory, F. Leek, G.P. Liney, Y. Liu, C.H. Clark. 2020 Tissue mimicking materials for imaging and therapy phantoms: a review. Physics in Medicine & Biology, 65(23), 23TR01.
[2] K. Wang, Y. Zhao, Y. H. Chang, Z. Qian, C. Zhang, B. Wang, M.J. Wang. 2016. Controlling the mechanical behavior of dual-material 3D printed meta-materials for patient-specific tissue-mimicking phantoms. Materials & Design, 90, 704-712.
[3] R. Murniati, E. Wibowo,M. Rokhmat, F. Iskandar, M. Abdullah. 2017. Natural rubber nanocomposite as human-tissue-mimicking materials for replacement cadaver in medical surgical practice. Procedia engineering, 170, 101-107.
[4] E. Bachmann, A. B. Rosskopf, T. Götschi, M. Klarhöfer, X. Deligianni, M. Hilbe, M.A. Fischer. 2019. T1-and T2*-mapping for assessment of tendon tissue biophysical properties: a phantom MRI study. Investigative Radiology, 54(4), 212-220.
[5] E.L. Madsen, J.A. Zagzebski, G.R. Frank. An anthropomorphic ultrasound breast phantom containing intermediate-sized scatterers, Ultrasound Med. Biol., 8 (4) (1982), pp. 381-392.
[6] M.K. Sun, J. Shieh, C.W. Lo, C.S. Chen, B.T. Chen, C.W. Huang, W.S. Chen. Reusable tissue-mimicking hydrogel phantoms for focused ultrasound ablation, Ultrason. Sonochem., 23 (2015), pp. 399-405
[7] E.L. Madsen, G.D. Fullerton. Prospective tissue-mimicking materials for use in NMR imaging phantoms, Magn. Reson. Imaging, 1 (3) (1982), pp. 135-141
[8] M.W. Kusk, J. Stowe, S. Hess, O. Gerke, S. Foley. 2023. Low-cost 3D-printed anthropomorphic cardiac phantom, for computed tomography automatic left ventricle segmentation and volumetry–A pilot study. Radiography, 29(1), 131-138.
[9] J. Greffier, Q. Durand, J. Frandon, S. Si-Mohamed, M. Loisy, , F.de Oliveira, D. Dabli. 2023. Improved image quality and dose reduction in abdominal CT with deep-learning reconstruction algorithm: a phantom study. European Radiology, 33(1), 699-710.
[10] S. Seeger, E. Elmoujarkach, N. Möller, C. Schmidt, M. Rafecas. 2022. 3D printed radioactive phantoms for Positron Emission Tomography. Transactions on Additive Manufacturing Meets Medicine, 4(S1), 640-640.
[11] S.J. Estermann, D.H. Pahr, A. Reisinger. 2022. Material design of soft biological tissue replicas using viscoelastic micromechanical modelling. Journal of the Mechanical Behavior of Biomedical Materials, 125, 104875.
[12] I. Develi. Experimental Investigation of the Heating Effects of 900 Mhz Cell Phones on Brain Phantom in Terms of Public Health.
[13] M. Higgins, S. Leung, N. Radacsi. 2022. 3D Printing Surgical Phantoms and their Role in the Visualization of Medical Procedures. Annals of 3D Printed Medicine, 100057.
[14] O.M. Bello, N.M. Nora, W.M.S.W. Hassana. Characterization of the Urethane Based Tissue Equivalent Substitute for Phantom Construction: Model Molding, XCOM and MCNPX Studies.
[15] A. Antoniou, C. Damianou. 2022. MR relaxation properties of tissue-mimicking phantoms. Ultrasonics, 119, 106600.
[16] R.B. Pearlin, R.S. Livingstone, A. Jasper, S.K.N. Keshava, G. Sridhar. 2022. Evaluation of radiation dose reduction and its effect on image quality for different flat-panel detectors. Journal of Medical Physics, 47(1), 73.
[17] J. Greffier, Q. Durand, J. Frandon, S. Si-Mohamed, M. Loisy, F. de Oliveira, D. Dabli. 2023. Improved image quality and dose reduction in abdominal CT with deep-learning reconstruction algorithm: a phantom study. European Radiology, 33(1), 699-710.
[18] D.M. Cash, T.K. Sinha, W.C. Chapman, H. Terawaki, M.I. Miga, Galloway Jr, R. L. (2003, May). Incorporation of a laser range scanner into an image-guided surgical system. In Medical Imaging 2003: Visualization, Image-Guided Procedures, and Display (Vol. 5029, pp. 269-280). SPIE.
[19] E. Momeni, F. Kazemi, P. Sanaei-Rad. 2022. Extraoral low-level laser therapy can decrease pain but not edema and trismus after surgical extraction of impacted mandibular third molars: a randomized, placebo-controlled clinical trial. BMC Oral Health, 22(1), 1-8.
[20] D. Hawkins, N. Houreld, H. Abrahamse. 2005. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Annals of the New York Academy of Sciences, 1056(1), 486-493.
[21] F.D.R.Guerra, C.P. Vieira, M.S. Almeida, L.P. Oliveira, A.A. de Aro, E.R. Pimentel. 2013. LLLT improves tendon healing through increase of MMP activity and collagen synthesis. Lasers in medical science, 28(5), 1281-1288.
[22] M.R. Hamblin. 2013. Can osteoarthritis be treated with light?. Arthritis research & therapy, 15(5), 1-2.
[23] M.R. Hamblin, T. Agrawal, M. de Sousa. (Eds.). 2016. Handbook of low-level laser therapy. CRC Press.
[24] H. Chung, T. Dai, S.K. Sharma, Y.Y. Huang, J. D. Carroll, M.R. Hamblin. 2012. The nuts and bolts of low-level laser (light) therapy. Annals of biomedical engineering, 40(2), 516-533.
[25] I.I. Geneva, B. Cuzzo, T . Fazili, W. Javaid . Normal Body Temperature: A Systematic Review. Open Forum Infect Dis. 2019 Apr 9;6(4):ofz032. doi: 10.1093/ofid/ofz032. PMID: 30976605; PMCID: PMC6456186.
[26] https://medlineplus.gov/ency/article/001982.htm
[27] A.R. Khaled, K. Vafai. 2003. The role of porous media in modeling flow and heat transfer in biological tissues. International Journal of Heat and Mass Transfer, 46(26), 4989-5003.
[28] R.C. Eberhart, A. Shitzer. (Eds.). 2012. Heat Transfer in Medicine and Biology: Analysis and Applications. Volume 2 (Vol. 2). Springer Science & Business Media.
[29] M. Fu, W. Weng, W. Chen, N. Luo. 2016. Review on modeling heat transfer and thermoregulatory responses in human body. Journal of thermal biology, 62, 189-200.
[30] M. M. Tung, M. Trujillo, J. L. Molina, M.J. Rivera, E.J. Berjano. (2009). Modeling the heating of biological tissue based on the hyperbolic heat transfer equation. Mathematical and Computer Modelling, 50(5-6), 665-672.
[31] S.L. Thomsen, J.E. Coad. (2007, February). Developing clinically successful biomedical devices by understanding the pathophysiology of the target tissue: insights from over 25 years at the microscope. In Thermal Treatment of Tissue: Energy Delivery and Assessment IV (Vol. 6440, pp. 9-23). SPIE.
[32] N. Bhardwaj, A.D. Strickland, F. Ahmad, L. Atanesyan, K. West, D.M. Lloyd. 2009. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology, 41(2), 168-172.
[33] M.S. Ferreira, J.I. Yanagihara. (2009). A transient three-dimensional heat transfer model of the human body. International Communications in heat and mass transfer, 36(7), 718-724.
[34] D. Gagnon, O. Jay, G.P. Kenny. 2013. The evaporative requirement for heat balance determines whole‐body sweat rate during exercise under conditions permitting full evaporation. The Journal of physiology, 591(11), 2925-2935.
[35] M. I. Gutierrez, S. A.Lopez-Haro, A.Vera, L. Leija. 2016. Experimental verification of modeled thermal distribution produced by a piston source in physiotherapy ultrasound. BioMed research international, 2016.
[36] https://phys.libretexts.org/@go/page/1587
[37] J. B. Leonard, K.R. Foster, T.W. Athley. 1984. Thermal properties of tissue equivalent phantom materials. IEEE Transactions on Biomedical Engineering, (7), 533-536.
[38] H.O. Durmuş, E.Ç.Arı, B. Karaböce, M.Y. Seyidov. 2021. Investigation of temperature effects of a 635 nm low power solid-state diode laser on agar phantom using two different thermocouples. Results in Optics, 5, 100142.
[39] B.L. Viglianti, et al. “Thresholds for thermal damage to normal tissues: An update,” International Journal of Hyperthermia, vol. 27, № 4, pp. 320-343, 2011, doi: 10.3109/02656736.2010.534527.
[40] https://energy-laser.com/guide-lines-for-treatment-with-laser-therapy.
|
