ABSTRACT
Using methods of physicochemical analysis (DTA, XRF, MSA, as well as density and microhardness measurements), the chemical interaction in the Ga2Sr-SrSe system
was studied and its T-x phase diagram was constructed. It has been established that the Ga2Sr-SrSe system is a quasi-binary section of the Sr-Ga-Se ternary system
and belongs to the eutectic type. In the Ga2Sr-SrSe system, there are limited regions of solid solutions based on the initial components at room temperature.
Microstructural analysis showed that at room temperature, solid solutions based on the Ga2Sr compound reach 5 mol % SrSe, and based on SrSe-3.5 mol % Ga2Sr. The
composition of the eutectic formed between the Ga2Sr and SrSe compounds is 25 mol % SrSe, at a temperature of 850°C. The lattice parameters were calculated as a
result of X-ray diffraction analysis of solid solutions (SrSe)1-x(Ga2Sr)x (x=0.01; 0.02; 0.03), respectively: for the SrSe compound, a=6.243 Å (SrSe)
and for alloys solid solutions, a=6.263 Å (1 % Ga2Sr), a=6.275 Å (2 % Ga2Sr), a=6.298 Å (3 % Ga2Sr). The temperature dependence of electrical
conductivity and thermo-EMF of solid solution alloys (SrSe)1-x(Ga2Sr)x (x=0.01; 0.02; 0.03) on composition was studied.
Keywords: system, phase, quasi-binary, eutectic, microhardness
DOI:10.70784/azip.1.2025137
Received: 17.03.2025
Internet publishing: 03.04.2025 AJP Fizika E 2025 01 en p.37-43
AUTHORS & AFFILIATIONS
1. Institute of Catalysis and Inorganic Chemistry named after M. Nagiyev, Ministry of Science and Education of the Republic of Azerbaijan
2. Baku State University, Baku, st. Z. Khalilov, 23.
3. Azerbaijan State University of Oil and Industry
4. Ganja State University
Graphics and Images
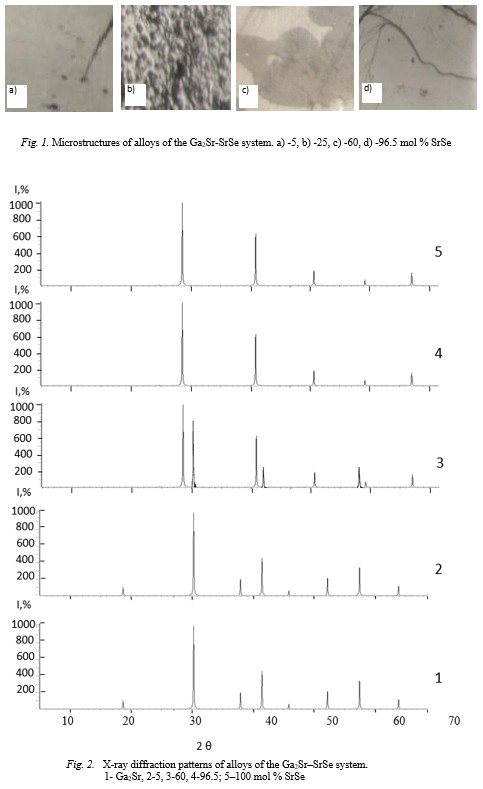
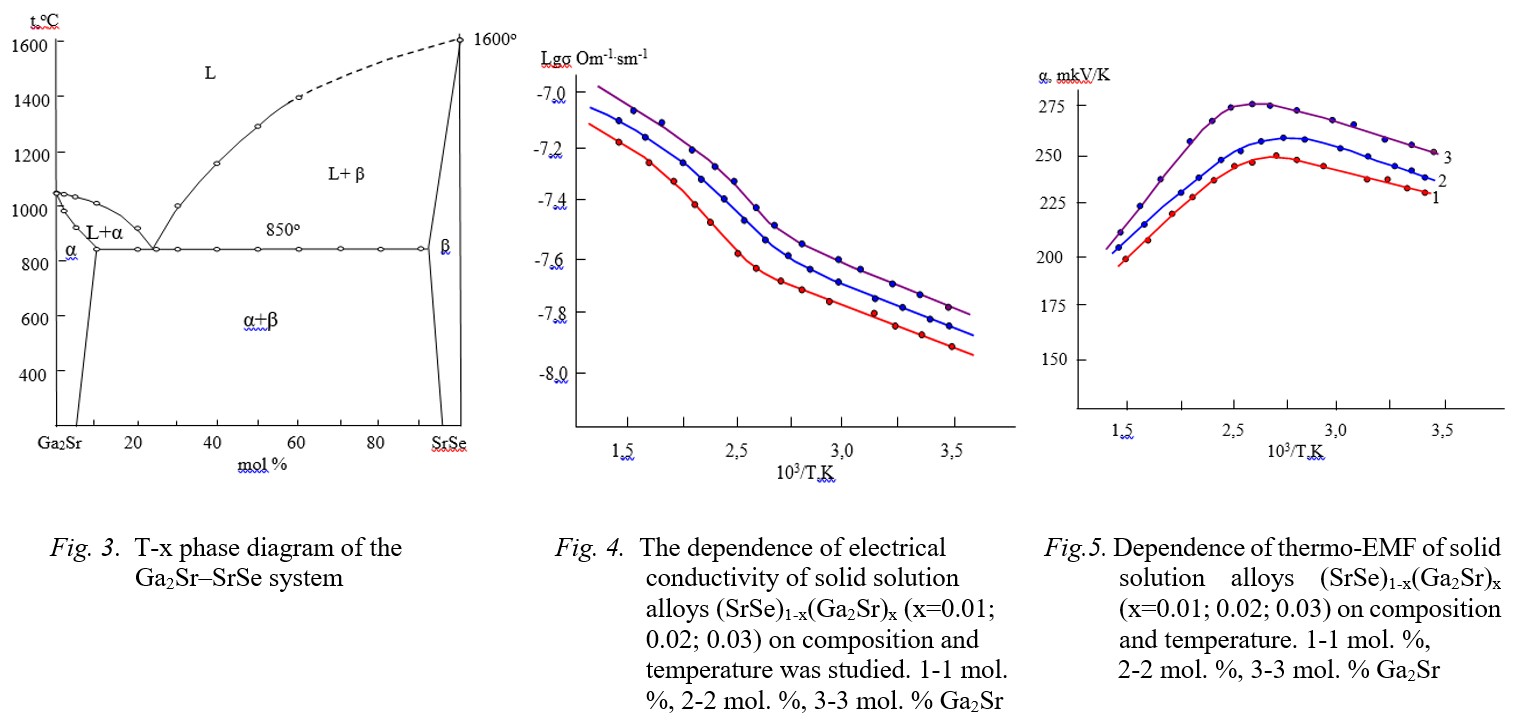
Fig.1-2 Fig.3-4-5
|
[1] Deli Jiang, Jie Li, Chaosheng Xing, Zhengyuan Zhang, Suci Meng, Min Chen. Two-Dimensional CaIn2S4/g-C3N4 Heterojunction Nanocomposite with Enhanced Visible-Light Photocatalytic Activities: Interfacial Engineering and Mechanism Insight. ACS Appl. Mater. Interfaces, 2015. V. 34. № 7. P. 19234–19242. doi: 10.1021/acsami.5b05118
[2] Jianjun Ding, Bin Hong, Zhenlin Luo, Song Sun, Jun Bao, Shen Gao. Mesoporous Monoclinic CaIn2S4 with Surface Nanostructure: An Efficient Photocatalyst for Hydrogen Production under Visible Light. J. Phys. Chem. C, 2014. V.118 . № 48. P. 27690–2769. https://doi.org/10.1021/jp508497a
[3] H. Hamada, I. Yoshida, Carkner Don, Wu X, M. Kutsukake, K. Oda. Inorganic EL devices with high-performance blue phosphor and application to 34-in. flat-panel televisions. Society for Information Display. Journal. 2008. V.16. № 12. P. 1183-1188. doi:10.1889/JSID16.12.1183
[4] D. Wauters, D. Poelman, R.L. Van Meirhaeghe, F. Cardon. Optical characterisation of SrS : Cu and SrS : Cu,Ag EL devices. J. Lumines. 2000. V. 91. № 1–6. https://doi.org/10.3390/ma3042834
[5] S. Tanaka, Y. Mikami, H. Deguchi, H. Kobayashi. White-light emitting thin-film electroluminescent devices with SrS:Ce,Cl/ZnS:Mn double phosphor layers. Jpn. J. Appl. Phys. Part 2 - Lett. 1986. V.25. L225–L227. doi 10.1143/JJAP.25.L225
[6] C.R. Wang, K.B. Tang, Q. Yang, C.H. An, B. Hai, G.Z. Shen, Y.T. Qian. Blue-light emission of nanocrystalline CaS and SrS synthesized via a solvothermal route. Chem. Phys. Lett. 2002. V. 351. P. 385-390. https://doi.org/10.1016/S0009-2614(01)01413-0
[7] Т.Н. Гулиев, П.Г. Рустамов, Н.И. Ягубов. Взаимодействие в системе СaSе-In2Sе3. Изв. АН СССР. Неорган. материалы. 1987. T.23. № 9. C.1447-1450.
[8] А.В. Кертман, О.И. Носов, О.В. Андреев. Реакции в системе CaS-In2S3. Журн. неорган. химии. 2002. T.47. № 1. С.126- 130. doi: 10.61413/Pfhg7389
[9] Т.Н. Гулиев, Н.И. Ягубов. Исследование взаимодействия в системе СaS-In2S3. Синтез и свойства неорганических соединений (Тематический сборник научных трудов) Баку. 1984. C.3-6.
[10] О.В. Андреев, Н.Н. Паршуков. Фазовые диаграммы системы CaS-Er2S3. Журн. неорган. химии. 2004. T.49. № 11. C.1763- 1766.
[11] О.В. Андреев, О.Ю. Митрошин, Н.А. Критокин, И.А. Разумкова. Фазовые равновесия в системе SrS-Ln2S3. Журн. неорган. химии. 2008. T.53. № 3. C. 440-444.
[12] А.В. Кертман, Н.В. Краеваб. Фазовые равновесия в системе SrS-Ga2S3. Журн. неорган. химии. 2010. T. 55. № 8. C. 1283-1286. doi: 10.61413/Pfhg7389
[13] Chiharu Hidaka, Nobuyasu Makabe, Takeo Takizawa. Determination of a pseudo-binary SrSe–Ga2Se3 phase diagram and single crystal growth of SrGa2Se4 compounds. Journal of Physics and Chemistry of Solids. 2003. V. 64, № 9–10. P. 1797-1800. https://doi.org/10.1016/S0022-3697(03)00096-9
[14] O.B. Андреев, A.B. Кертман, В.Г. Бамбуров. Фазовые равновесия в сис¬темах La2S3-SrS и Nd2S4-SrS. Журн. неорган. xимии. 199. T. 36. № 1. C. 253-257.
[15] О.В. Андреев, О.Ю. Митрошин, И.А. Разумкова. Фазовые равновесия в системах Sc2S3-SrS и Sc2S4-BaS. Журнал неорган. химии. 2008. T. 53. № 2. C. 366-369.
[16] W. Klee, H. Schofer. Duratellung und krystallstructur. Von SrIn2Se4 and CaIn2Se4 Rib. Chim. miner. 1979 . V.16. P.463-466.
[17] Б.Г. Тагиев, О.Б. Тагиев, Р.Б. Джаббаров, Н.Н. Мусаева, У.Ф. Касимов. Фотолюминесценция в соединениях Ca4Ga2S7:Ce3+ и Ca4Ga2S7:Pr3+ Неорган. материалы. 2000. T. 36. № 1. C. 3-6. doi: 10.61413/Pfhg7389
[18] A.N. Georgobiani, B.G. Tagiev, S.A. Abushov, O.B. Tagiev, Zhen Xu, and Suling Zhao. Photo–and thermoluminescence of Eu, BaGa2Se4, Eu,BaGa2Se4, Eu,Ce crystals. Inorg. mat. 2008. V. 44. № 2. P. 110 – 114. ISSN 1996-3955
[19] H. Najafov, A. Kato, H. Toyota, K. Iwat, A. Bayramov and S. Lida. Effekt Ce co-doping on CaGa2S4:Eu phosphor: II. Thermoluminescence. Japn. J. Appl. Phys. 2002. V. 44. P. 2058 – 2065. doi:10.1143/JJAP.41.2058
[20] J.E. Van Haecke, P.F. Smet, D. Poelman. The influence of source powder composition on the electroluminescence of Ca1-xSrxS:Eu thin films. Spectroc. Acta Pt. B-Atom. Spectr. 2004. V. 59. P. 1759-1764.
[21] Y.N. Yaqubov, İ.İ. Əliyev, F.İ. İsmayılov. CaIn2-CaTe sisteminin faza diaqramı. Az. Kimya jurnalı. 2014. № 1. S.70-74.
[22] N.İ. Yaqubov, İ.İ Əliyev. Ca3In-CaTe sisteminin faza diaqramı BDU Xəbərləri 2014. № 3. C. 18-23.
[23] İ.İ. Əliyev, N.İ. Yaqubov, N.A. Məmmədova. Ca3In-CaSe sisteminin fiziki-kimyəvi tədqiqi. Kimya Problemləri. 2013. № 4. S.432-436.
[24] И.И. Алиев, Р.Л. Мусаева, Н.И. Ягубов, Ф.М. Садыгов, Ф.И. Исмаилов. Характер взаимодействия в системе InSe-CaSe. Журн. неорган. химии. 2009. T.54. № 8. C. 1398-1400.
[25] Диаграмма состояния двойных металлических систем: Справочник: В 3 т.: Т.2 / Под. Общ. ред. Н.П. Лякишева. –М.: Машиностроение. 1997-1024 с.
[26] Диаграмма состояния двойных металлических систем: Справочник: В 2 т.: Т.3 / Под. Общ. ред. Н.П.Лякишева. –М.: Машиностроение. 2000-448 c.
[27] N.B. Kolomiets. Measurement of thermoelectromotive force and resistivity in the temperature range from 20 to 1900oС. Factory laboratory. 1962. T.28. № 2. P. 238-240.
[28] A. Okhotin, N. Pushkarskiy, R. Borovikova, R. Smirnov. Methods of investigation of thermoelectric properties of semiconductors. M.: Atomnzdat. 1969.175 p.
|
